Principle of cosmogenic nuclides
Cosmic radiation
Our planet is permanently impacted by a particle flow from space called cosmic rays.
Discovered in the early 20ème In this century, two types of radiation are distinguished:
. a primary radiation mainly protons (85%), alpha particles (Helium nuclei, 12%). The remaining part (3%) consists of heavy nuclei and electrons. The most commonly accepted origin of cosmic rays is the acceleration of interstellar plasmas by shock waves, such as those generated by a supernova explosion. While the most energetic particles in cosmic radiation originate outside the Solar System, energetic particles are also produced by the Sun. The solar and galactic components differ in their compositions, energy distributions and intensities as a function of time.
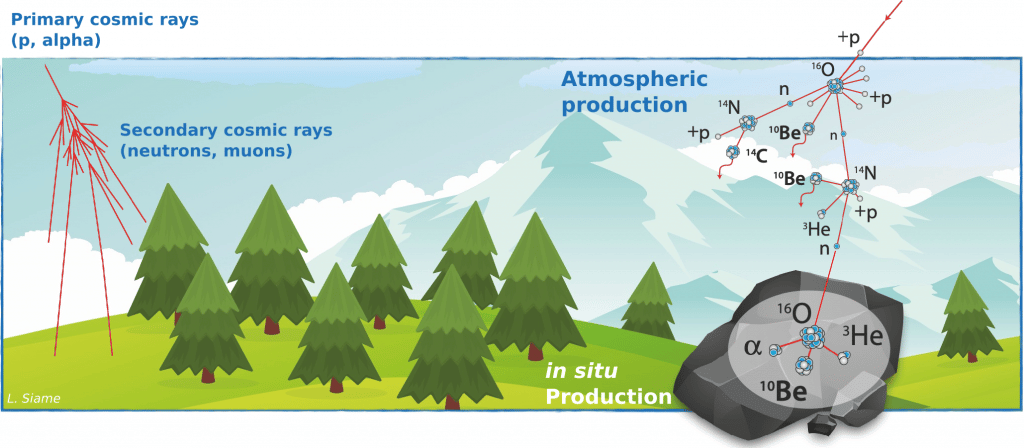
. a secondary radiation resulting from the impacts of primary radiation particles on the constituents of the atmosphere which leads to the formation of cosmic sprays (neutrons and muons). When the primary cosmic ray particles enter the Earth's atmosphere, a cascade of nuclear reactions will occur with the atoms that make up the atmosphere. Their energy is thus dissipated in these nuclear reactions. The flux and energy of the primary and secondary particles (mainly neutrons) produced in the nuclear cascades decrease rapidly according to an exponential law depending on the thickness of the atmosphere.
Cosmogenic nuclides
Lhe very high energy of cosmic rays enables them to trigger nuclear reactions when they impact the nuclei of the elements that make up the atmosphere and the earth's subsurface.
Most of the reactions take place in the upper atmosphere. At sea level, only 0.00003% of primary protons remain. Similarly, almost all secondary radiation particles dissipate their energy in the atmosphere. Only about 0.1% of the secondary particles reach the ground with enough energy to induce nuclear reactions in the minerals making up the rocks of the Earth's crust exposed at the surface.
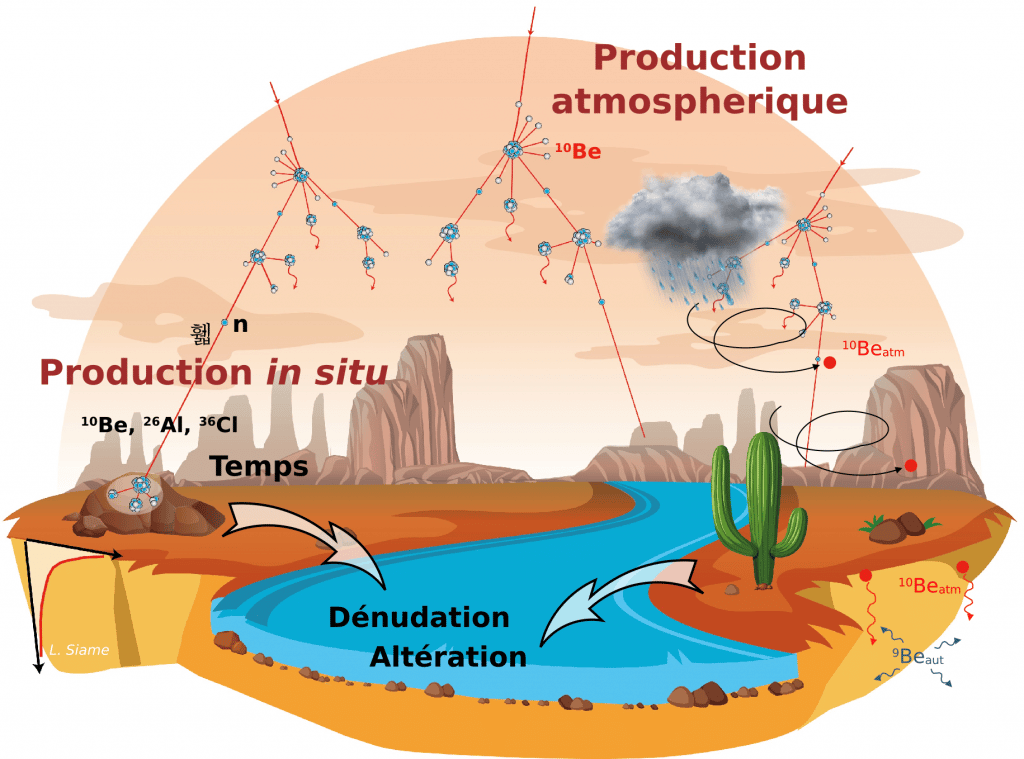
Atomic nuclei that are produced by nuclear reactions between target atoms in the Earth's atmosphere (atmospheric production) or minerals in the rocks that make up the Earth's crust (in situ production, up to a few metres below the surface) and cosmic ray particles (primary or secondary) are commonly referred to as cosmogenic nuclides.
These nuclear reactions are mostly spallation reactions, i.e. reactions in which the impacting particle (mostly a neutron) has enough energy to pull constituent particles (neutrons and protons) from the nucleus target atomic without being captured, and thus leaving as a residue a nucleus of a different chemical species since both the atomic number (number of protons) and the mass number (number of protons + number of neutrons) are lower than that of the original target nucleus.
In the atmosphere the cosmogenic nuclide production rate decreases with decreasing altitude, due to the particle flux decreasing with the thickness of the atmosphere. In the Earth's crust, the rate of cosmogenic nuclide production decreases as a function of the depth of material traversed according to an exponential law.
As an example, in situ cosmogenic nuclide production is of the order of tens to hundreds of atoms per gram of rock per year at the surface, resulting in measured concentrations of the order of thousands (10³) to millions (106) of atoms per gram of rock. By way of comparison, the total number of atoms in a gram of rock is several trillion billion (1022). These very low abundances in the samples studied require the use of specific preparation and measurement techniques.
Applications in geochronology
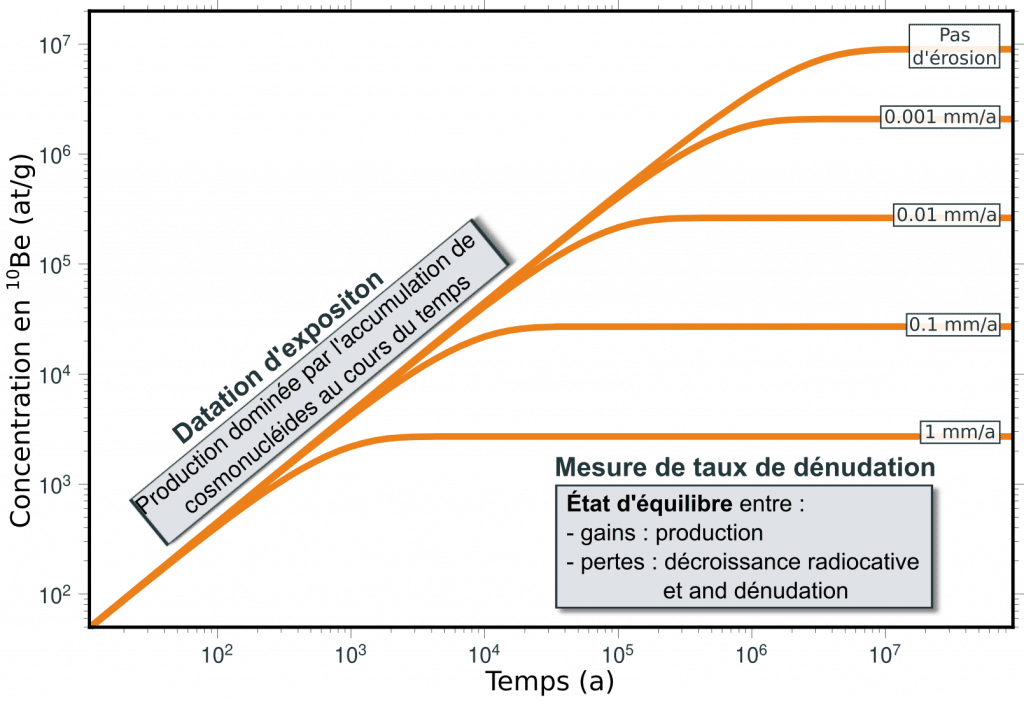
The accumulation of in situ produced cosmonuclides in surface and subsurface rocks over time depends on the duration of exposure, the rate of denudation of the surface in response to erosion processes and the radioactive decay of the nuclide.
Thus, an exposed surface will initially see its concentration increase in proportion to the local production rate until it reaches a state of equilibrium where gains and losses compensate, with a concentration inversely proportional to the rate of denudation of the surface. Concentrations measured in objects as varied as glacial moraines and polishes, alluvial terraces and fans, or sediments can be interpreted in terms of the age of these objects or the rate of erosion (or both).